In a living cell, newly synthesized proteins must flex to gain their functions. This folding can be spontaneous, or with the help of a class of proteins called “companions”. Hsp90 and Hsp70 (also called DnaK in bacteria) are conserved chaperones from bacteria to humans. The model implemented a few years ago shows the cooperation of the two escorts in the laboratory Collapsible form pillars.
To understand the importance of this cooperation He lives In bacteria, the scientists made mutations in Hsp90 in the region of its interaction with DnaK. in home Shewanella oneidensiswhich are aquatic bacteria, they notice them He lives Disruption of the interaction between Hsp90 and DnaK prevents growth during heat stress. This growth defect is associated with a known substrate of Hsp90 called TilS. Thus, they concluded that correct folding of TilS requires cooperation between Hsp90 and DnaK via a direct interaction between the two chaperones. in home Escherichia coli, Mutations that nullify the interaction between Hsp90 and DnaK result in no production of a toxin, colibactin, known to be implicated in the development of cancers in humans. These results demonstrate that cooperation between Hsp90 and DnaK is essential in coli bacteria To produce colibactin, the synthesis of which indirectly requires Hsp90, possibly by folding of one or more proteins of this biosynthesis pathway.
This work reveals for the first time the importance of the cooperation between Hsp90 and DnaK in bacteria, both in stress processes and in the production of toxins by bacteria.
In eukaryotes, cooperation between Hsp90 and DnaK is also known and includes other partners. Thus, the model of cooperation between bacterial Hsp90 and DnaK has evolved towards a more complex system, thus adapting to the challenges of the eukaryotic cell and its proteins.
It is already known that Hsp90 is involved in the pathogenicity of some bacteria. Given the importance of the cooperation between Hsp90 and DnaK in pathogenicitycoli bacteria, This study could open up the development of new molecules to reduce bacterial virulence while multi-resistant bacteria represent a major public health problem.
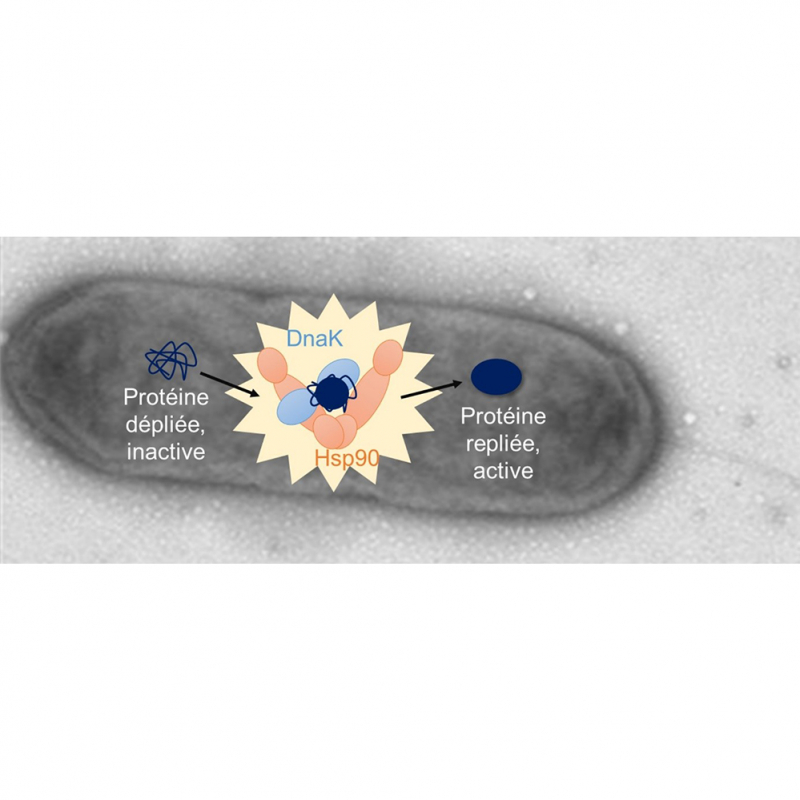
picture: Cooperation between Hsp90 and DnaK chaperones is important for bacterial adaptation to heat stress S.oneidensis And to produce poison in coli bacteria.
Some proteins, in order to fold properly, require the intervention of the DnaK and Hsp90 chaperones that cooperate via a direct interaction. By separating the activities of Hsp90 and DnaK by point mutations on Hsp90, the scientists demonstrated that this cooperation has real significance in bacteria. He lives. This cooperation is particularly necessary to adapt to the heat stress of environmental bacteria. S.oneidensisas well as the virulence of the pathogen coli bacteria. in home coli bacteriathe conjugated activity of DnaK and Hsp90 indirectly allows the biosynthesis of colibactin, a genetic toxin responsible for double-strand breaks in the DNA of the target cell.
To find out more:
Uncoupling the activities of Hsp90 and DnaK chaperones revealed the importance of their cooperation in bacteria in vivo.
Corteggiani M, Bossuet-Greif N, Nougayrède JP, Byrne D, Ilbert M, Dementin S, Giudici-Orticoni MT, Méjean V, Oswald E, Genest O.
Proc Natl Acad Sci US A. Sep 13, 2022 doi:10.1073/pnas.2201779119.

“Hardcore beer fanatic. Falls down a lot. Professional coffee fan. Music ninja.”






More Stories
A documentary film denouncing the destruction of the planet
Robert Sovi Institute for Occupational Health and Safety Research
Starliner's first manned flight in May